Trinetra Mitra

Title: Evaluating the impact of british society of rheumatology guidelines on pregnancy outcomes in rheumatoid arthritis: A single centre experience
Abstract
Purpose:
Rheumatoid arthritis (RA) increases the risk of adverse pregnancy outcomes, particularly if RA is active during pregnancy. British Society for Rheumatology (BSR) guidelines, first published in 2016, support the use of disease-modifying anti-rheumatic drugs (DMARDs), including biologic (b)DMARDs, during pregnancy. Despite these and other international guidelines, caution persists on use of bDMARDs in RA pregnancy. Therefore, we studied the impact of the 2016 BSR pregnancy guideline on real world bDMARD use and RA pregnancy outcomes.
Methods:
We retrospectively reviewed RA pregnancy outcomes from the University College London Hospital (UCLH) obstetric rheumatology clinic from January 2013–May 2023. Disease severity was classified as remission/low, moderate, or severe based on pre-set criteria (i.e, joint counts, medication changes). From April 2019 onwards, UCLH transitioned to a fully electronic medical record allowing complete data capture. We thus analysed individuals delivering at UCLH post-April 2019 as a sub-cohort to additionally evaluate incidence of gestational diabetes (GDM) and pre-eclampsia.
Results:
A total of 105 RA pregnancies were identified. Median maternal age was 35 years, median birthweight 3285g, and median gestational age at delivery 276 days, with 8.7% delivered preterm and 20% small for gestational age (SGA). Of those with recorded activity in the 3rd trimester (n=89/105), 81% achieved remission/low activity and 51% had a postpartum flare. Overall, 11.4% discontinued a bDMARD at some point in pregnancy, while 10.5% continued a bDMARD throughout all three trimesters and 78.1% [IG1] had no biologic use.
Analysis pre- and post-introduction of the 2016 BSR guideline demonstrated an increase in TNF inhibitor bDMARD use from 11% to 22%, and in non-TNF inhibitor bDMARD use from 0% to 4%. Steroid use decreased from 50% to 34%. In contrast, SGA births increased from 16% to 22%, and preterm births rose from 0% to 12%.
In the post-April 2019 (n=40) sub-cohort, 20% developed GDM, 2.5% pre-eclampsia, and 23% conceived using assisted reproduction techniques. Of those with recorded disease activity in the 3rd trimester (n=36/40), 88.8% achieved remission/low activity, with only 30% flaring postpartum.
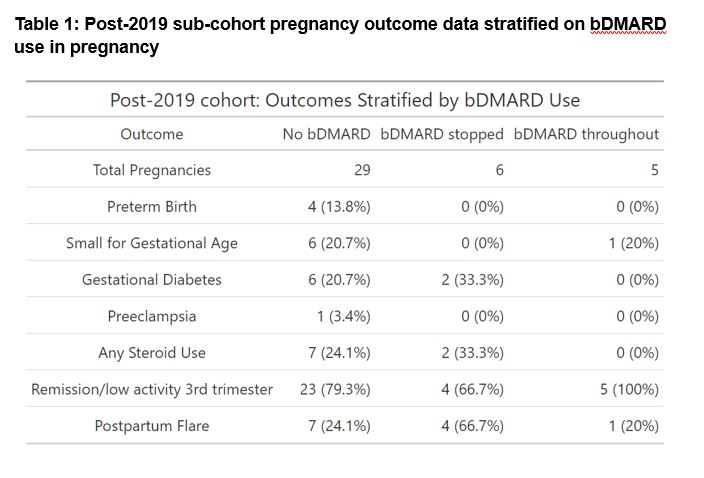
Table 1 summarises the post-2019 sub-cohort outcomes of individuals not on bDMARDS, compared to those on bDMARDs who either stopped or continued therapy in pregnancy. It illustrates that maternal complications are highest in those who discontinue bDMARDs in pregnancy (i.e., least likely to achieve remission/low disease activity in the 3rd trimester; and highest rates of steroid use, GDM and postpartum flare). Fetal complications however were highest in those mothers not on bDMARDs (i.e., highest rates of SGA and preterm birth).
Conclusions:
This decade-long, real-world study highlights increasing bDMARD use following the introduction of the 2016 BSR guidelines. Importantly, continuation of bDMARDs throughout pregnancy was associated with improved maternal-fetal outcomes and supports their continuation.